| Gas | Formel | Mr in g/mol |
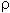 0 in g/dm3 0 in g/dm3(0°C,po) |
d (Luft=1) | V0in dm3/mol |
| Luft (trocken) | n.a. | 28,96 | 1,2928 | 1,000 | 22,40 |
| Helium | He | 4,00260 | 0,1785 | 0,1381 | 22,42 |
| Neon | Ne | 20.179 | 0,8999 | 0.6961 | 22,42 |
| Argon | Ar | 39,948 | 1,7839 | 1.3799 | 22,39 |
| Krypton | Kr | 83,8 | 3,744 | 2,896 | 22,25 |
| Xenon | Xe | 131,29 | 5,896 | 4,561 | 22,27 |
| Wasserstoff | H2 | 2,01588 | 0,08987 | 0,06952 | 22,43 |
| Sauerstoff | O2 | 31,9988 | 1,42895 | 1,1053 | 22,39 |
| Ozon | O3 | 47,9982 | 2,1415 | 1,6565 | 22,41 |
| Stickstoff | N2 | 28,0134 | 1,25046 | 0,9672 | 22,40 |
| Chlor | Cl2 | 70,906 | 3,214 | 2,4861 | 22,06 |
| Fluor | F2 | 37,9968 | 1,696 | 1,312 | 22,40 |
| Kohlenmonoxid | CO | 28,0104 | 1,2500 | 0,9669 | 22,41 |
| Kohlendioxid | CO2 | 44,0098 | 1,9769 | 1,5292 | 22,26 |
| Stickstoffmonoxid | NO | 30,0061 | 1,3402 | 1,0367 | 22,39 |
| Distickstoffoxid | N2O | 44,0128 | 1,9804 | 1,5319 | 22,22 |
| Schwefeldioxid | SO2 | 64,0588 | 2,9262 | 2,2635 | 21,89 |
| Chlorwasserstoff | HCl | 36,461 | 1,6392 | 1,2679 | 22,24 |
| Bromwasserstoff | HBr | 80,912 | 3,6443 | 2,8189 | 22,20 |
| Iodwasserstoff | HI | 127,9124 | 5,789 | 4,478 | 22,10 |
| Ammoniak | NH3 | 17,0304 | 0,7714 | 0,5967 | 22,08 |
| Phosphin | PH3 | 33,9975 | 1,5307 | 1,1840 | 22,21 |
| Wasser (Dampf) | H2O | 18,0153 | (0,804) | 0,622 | 22,41 |
| Schwefelwasserstoff | H2S | 34,0758 | 1,5385 | 1,1901 | 22,15 |
| Selenwasserstoff | H2Se | 80,9758 | 3,6643 | 2,8344 | 22,10 |
| Methan | CH4 | 16,0428 | 0,7168 | 0,5545 | 22,38 |
| Ethan | C2H6 | 30,0696 | 1,3566 | 1,0494 | 22,17 |
| Propan | C3H8 | 44,0962 | 2,0096 | 1,5545 | 21,94 |
| Ethen | C2H4 | 28,0536 | 1,2604 | 0,9749 | 22,26 |
| Ethin | C2H2 | 26,0379 | 1,1747 | 0,9086 | 22,17 |
d= relative Dichte
Vo = Molvolumen
1 Liter entspreicht per Definition 1dm3.
Mr = relative Molmasse

